Research
Numano Lab.
Department of Environmental and Life Sciences
Electronics-Inspired Interdisciplinary Research Institute
Rika Numano
Project Associate Professor;
numano@eiiris.tut.ac.jp;
http://www.tut.ac.jp/english/schools/faculty/ens/677.html
Circadian rhythms, Period1, Transgenic mice, TOYOHASHI probe, pacemaker neuron,, LiGluR, azobenzene, MAG, a water-in-oil droplet electroporation, Transfection
would like manipulate neural activity and physiological reaction by our original electrical probe, photo-switched nanomachines and transfection method both in vitro and in vivo. For example, the pacemaker neuron activity of circadian rhythms in the SCN can be manipulated by stimulating the SCN target circuit, which investigates the mechanism to control rhythms of the whole body, and shows how to maintain and administer the health life with normal rhythms.
1.Analysis of pacemaker neurons in mammalian circadian rhythms using Per1 Tg mice and TOYOHASHI probe
The biochemical, physiological and behavioral processes are under the control of internal clocks with the period of approximately 24 hr, circadian rhythms. The expression of mouse Period1 (mPer1) gene oscillates autonomously in the suprachiasmatic nucleus (SCN). Per1 is an indispensable member of the central clock system to maintain the autonomous oscillator. I constructed Per1:luc Tg mice and rats in which firefly luciferase was rhythmically expressed under the control of the mouse Per1 promoter in order to monitor mammalian circadian rhythms by Per1 rhythmic expression.
I performed functional analysis of circadian pacemaker neurons in the SCN by TOYOHASHI original electrophysiological probe with nano-size electrode other than Per1 expressional rhythms.
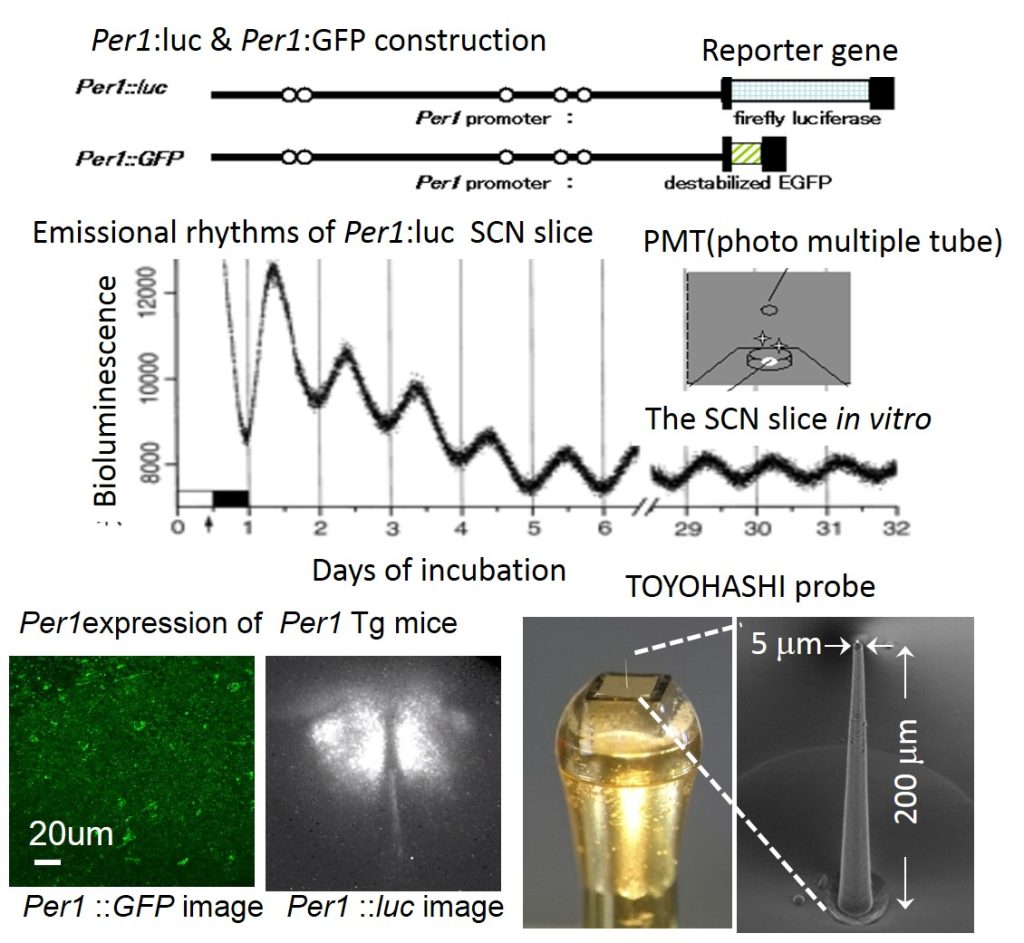
fig.1Emissional circadian rhythms in the SCN of Per1 Tg and TOYOHASHI probe
Rhythmic emission from the cultured SCN slices of Per1:luc Tg mouse persisted for up to some months in vitro. Per1 expression could be observed in one cell level by the SCN slice of Per1:GFP Tg. Electric activity of the SCN pacemaker neurons could be detected in one cell level by TOYOHASHI probe with nano-several micro meter diameter probe.
2. Manipulation of neural activity under optical control by bionanomachine.
I recognize intact biostructure ionotropic glutamate receptors (iGluR6) as machinery, which is normally expressed in synaptic neural processes in mammalian brain. To control any neural activity remotely and reversibly, photoswitchable nanomachine LiGluR were developed based on iGluR6 and operated using photoiosomerizable new chemicals, MAG. Two iGluR6 mutants could be photo-switched using a series of maleimde-azobenzene-glutamate (MAG) compounds, which dangled 2R,4R-allyi glutamate (G) from a linker containing the photoisomerizable azobenzene (A) that was attached to the introduced cysteines via maleimide (M). Three kinds of MAGs were examined at cysteine positions around the “mouth” of the ligand binding domain “clamshell” from geometry. LiGluR: opening in UV light and closing in visible light by all MAGs. In neural cells with LigluR, action potentials were optimally evoked and extinguished by UV and visible light, respectively. These photo-switched nanomachines could manipulate neural activity under optical control both in vitro and in vivo.
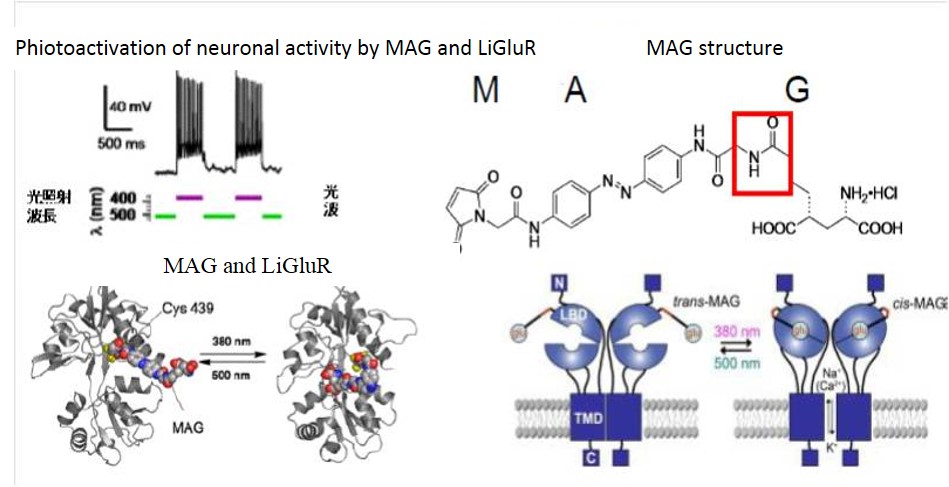
fig.2 Phiotoactivation of neuronal activity by MAG and LiGluR
LiGluR is based on the reversible photoisomerization of maleimide-azobenzene-glutamate (MAG) between its trans configuration under 500 nm light and its cis configuration under 380 nm light. MAG is covalently attached by the maleimide moiety to a cysteine introduced into the ligand-binding domain (LBD) of the receptor. MAG binding under 380 nm light activates the receptor and opens its cation-selective channel, resulting in membrane depolarization.
3. Novel Parallelized Electroporation by Electrostatic Manipulation of a Water-in-oil Droplet as a Microreactor
Electroporation is the most widely used transfection method for delivery of cell-impermeable molecules into cells. We developed a novel gene transfection method, water-in-oil (W/O) droplet electroporation, using dielectric oil and an aqueous droplet containing mammalian cells and transgene DNA. When a liquid droplet suspended between a pair of electrodes in dielectric oil is exposed to a DC electric field, the droplet moves between the pair of electrodes periodically and droplet deformation occurs under the intense DC electric field. This method has several advantages over conventional transfection techniques, including co-transfection of multiple transgene DNAs into even as few as 1000 cells, transfection into differentiated neural cells, and the capable establishment of stable cell lines. This technique will lead to the development of cell transfection methods for novel regenerative medicine and gene therapy.
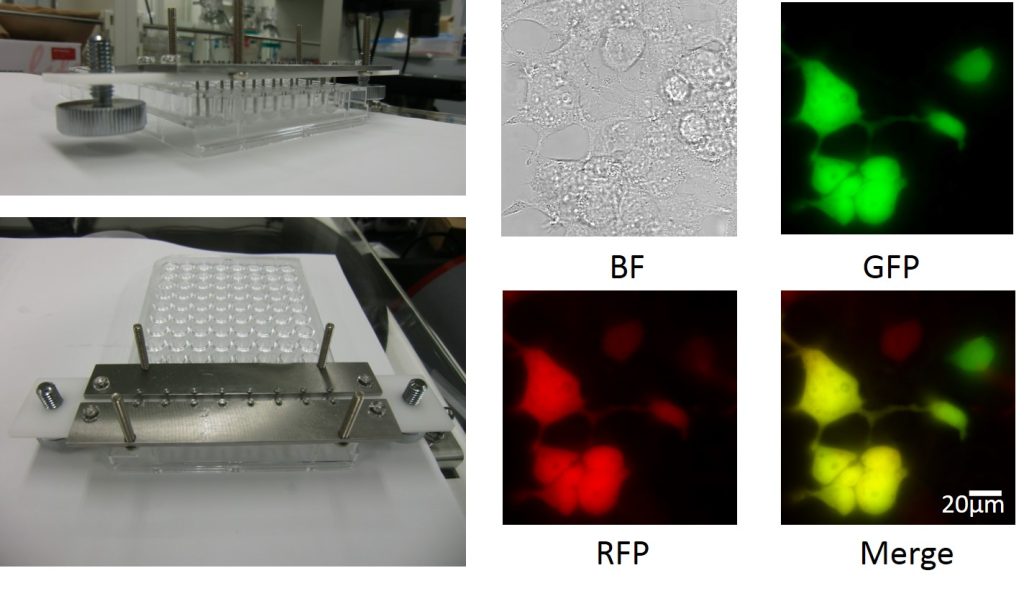
fig.3 Water-in-oil (W/O) droplet electroporation
Image of the parallel W/O droplet electroporation electrode for the 8-well string of disposable 96-well plates and HEK293cells transfected fluorescent protein plasmid by W/O droplet electroporation. BF: Bright field image, GFP: Green Fluorescent Protein image RFP: Red Fluorescent Protein image, Merge: Merge image of GFP and RFP





